Interdisciplinary Case Report
CDOCS
MAY 4, 2021
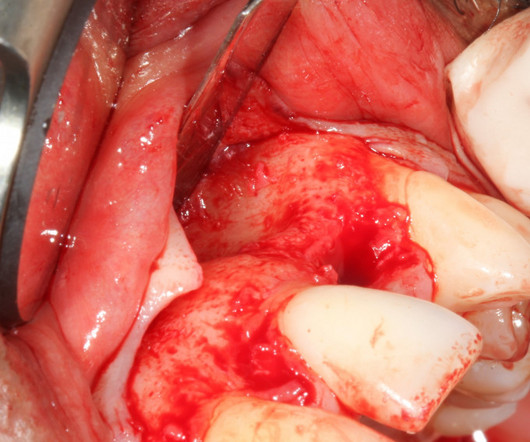
Without this additional skill set and post graduate training I simply could not deliver the results that my patients deserve. We need to recognize how clinical dentistry has become intertwined with digital technologies to synergistically assist us in providing enhanced diagnosis with safe and predictable treatment outcomes. &



















Let's personalize your content