Outcomes of Guidelines from Health Technology Assessment Organizations: A Systematic Mixed Studies Review [Systematic review, meta-analysis, or scoping review]
Annals of Family Medicine
NOVEMBER 20, 2024
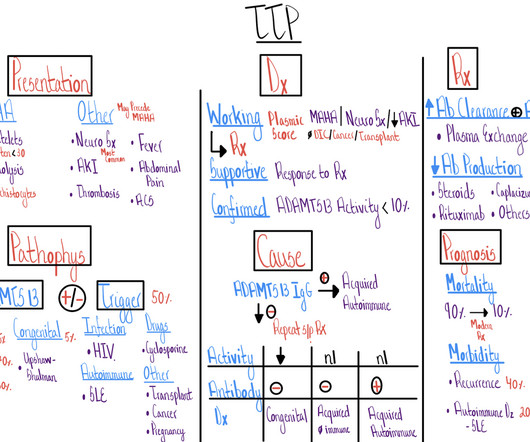
Context: Health Technology Assessment (HTA) organizations determine the value of health technologies such as medical devices, lab tests, or medications. positive, neutral, and negative impact) are presented for 17 types of outcomes. Twenty-nine types of outcomes are qualitatively documented.















Let's personalize your content