Journey of a pill
Canadian Family Physician
APRIL 14, 2025
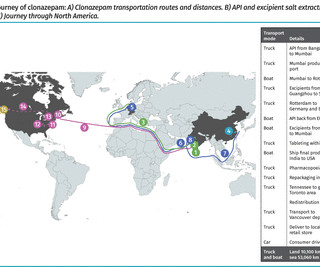
The product is then shipped back to India for the next manufacturing stages. After shipment through Europe and Asia, the journey continues through several locations within the United States, specifically New Jersey, for the final stages of manufacturing. Quality testing and stocking occur elsewhere, such as within the European Union.





























Let's personalize your content