Clinical Reasoning Corner: Illness Script
The Clinical Problem Solvers
FEBRUARY 27, 2022
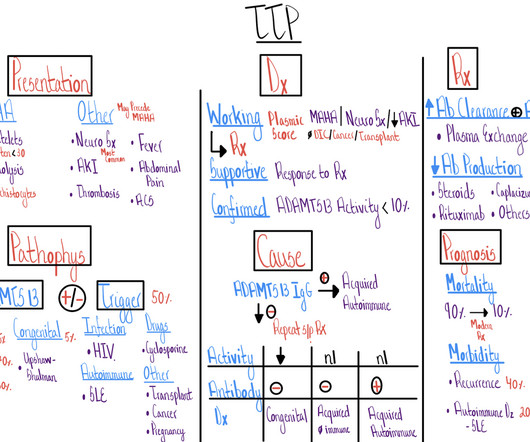
An illness script is a clinical reasoning tool used by clinicians to collect and retrieve clinically relevant information about a particular condition. lab tests, imaging, and/or pathology). Sometimes this can be challenging because there is so much information available and many diseases have overlapping features (e.g.,



















Let's personalize your content