The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
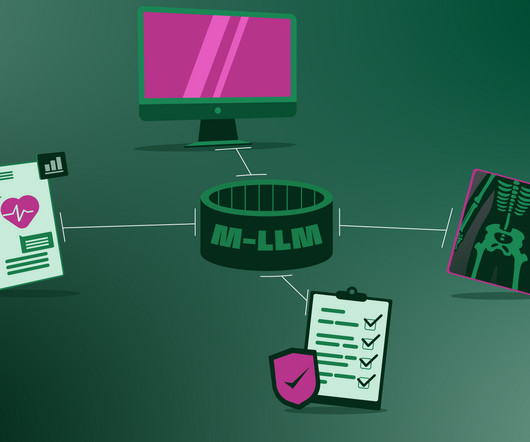
Although this revolution has been brewing for years, the past few months marked a major change , as algorithms finally moved out of the specialized labs and into our daily lives. Current medical AIs only process one type of data, for example, text or X-ray images. However, medicine, by nature, is multimodal as are humans.


















Let's personalize your content