AAFP Represents Members During 2025 Legislative Session
Alabama Academy of Family Physicians
MAY 15, 2025
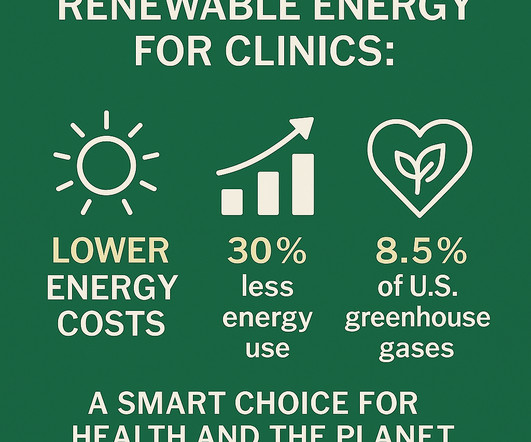
During the session, the Alabama Academy of Family Physicians actively represented the interests of its members on a range of healthcare initiatives. These funds could be utilized for various purposes, including direct care, operational expenses, and facility maintenance or upgrades.




















Let's personalize your content