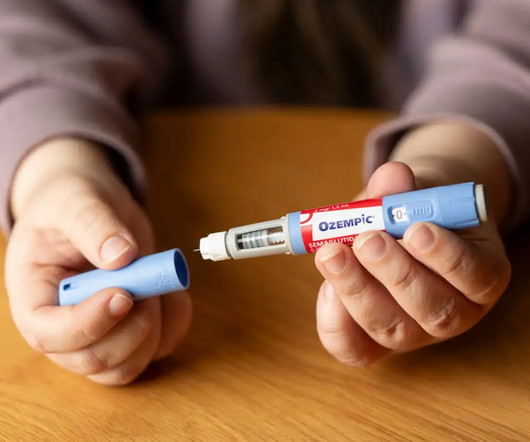
How to Get Ozempic & GLP-1’s Covered by Insurance (or Save Big with Plum Health Direct Primary Care): The Ultimate Guide You Can’t Afford to Miss!
Plum Health
AUGUST 30, 2024
Managing diabetes—especially as you get older—can be challenging physically, emotionally, and financially. Everything You Need to Know About Costs and Coverage Patient is inquiring about healthcare. If you have commercial insurance, you may be eligible to pay as little as $25 for Ozempic using a savings card from the manufacturer.















Let's personalize your content