Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
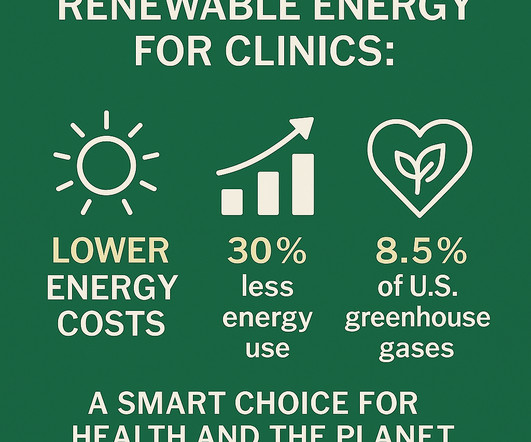
For instance, the UK government’s initiative to install solar panels on National Health Service sites is projected to save each site up to £45,000 annually, amounting to approximately £13 million in total savings per year. The breadth of services provided by modern day medicine is profound.












Let's personalize your content