The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
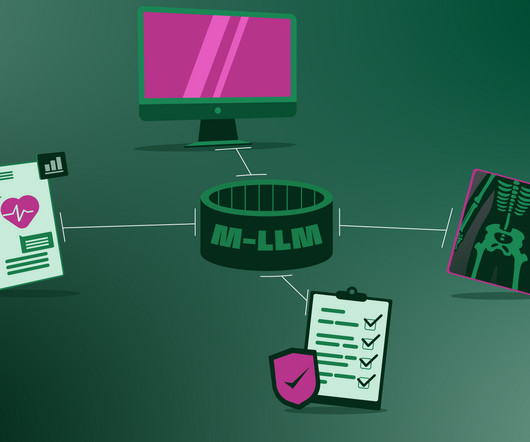
To diagnose and treat a patient, a healthcare professional listens to the patient, reads their health files, looks at medical images and interprets laboratory results. ” Patient points to lower abdomen. M-LLM (Translating for Specialist): “The patient is pointing to the lower abdomen.”





















Let's personalize your content