The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
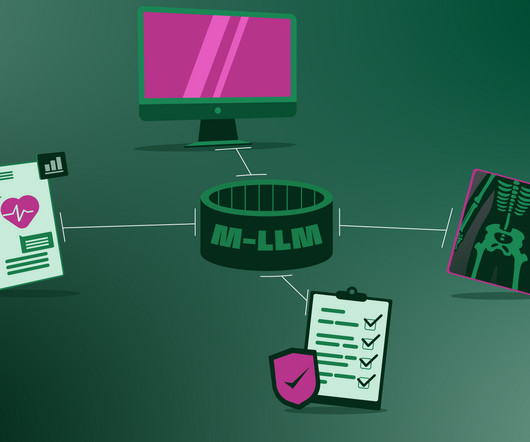
It will break language barriers These M-LLMs will easily facilitate communication between healthcare providers and patients who speak different languages, translating between various languages in real time. No single company will come up with such software because they don’t have access to the AI data developed by individual companies.
















Let's personalize your content