The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
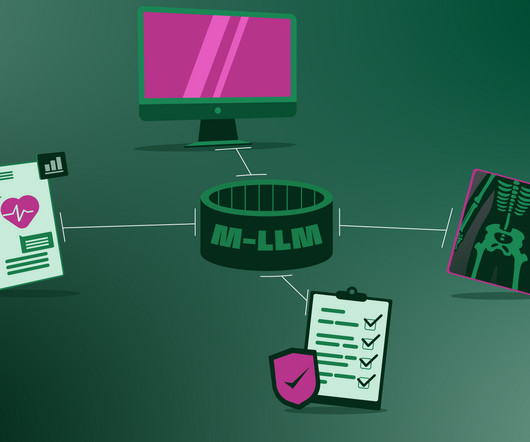
It will break language barriers These M-LLMs will easily facilitate communication between healthcare providers and patients who speak different languages, translating between various languages in real time. AI used in the hospital. Just as we’ve seen how live translation works with ChatGPT-4o.











Let's personalize your content