The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
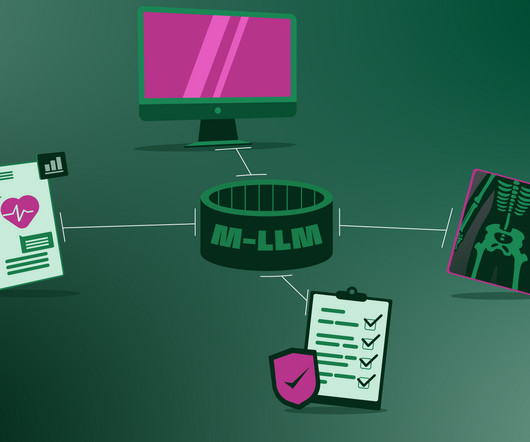
Finally, the arrival of interoperability can connect and harmonise various hospital systems An M-LLM could serve as a central hub that facilitates access to various unimodal AIs used in the hospital, such as radiology software, insurance handling software, Electronic Medical Records (EMR), etc. AI used in the hospital.











Let's personalize your content