The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
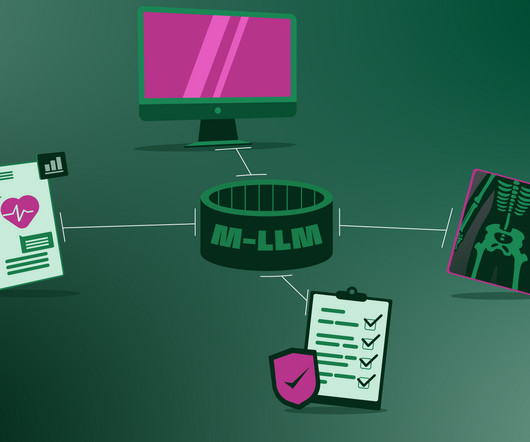
Large language models will soon find their way in to everyday clinical settings , simply because the global shortage of healthcare personnel is becoming dire and AI will lend a hand with tasks that do not require skilled medical professionals. Current medical AIs only process one type of data, for example, text or X-ray images.













Let's personalize your content