The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
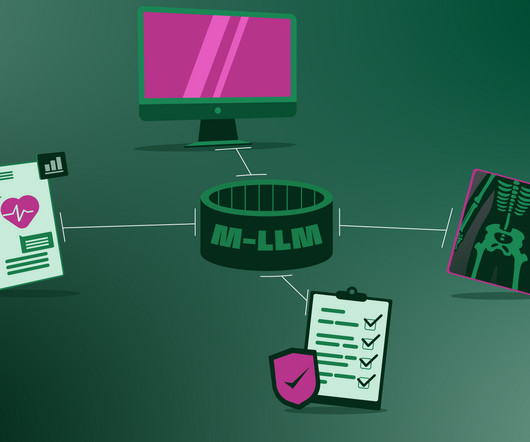
Finally, the arrival of interoperability can connect and harmonise various hospital systems An M-LLM could serve as a central hub that facilitates access to various unimodal AIs used in the hospital, such as radiology software, insurance handling software, Electronic Medical Records (EMR), etc.










Let's personalize your content