The Healthcare Vision of ChatGPT-4o and Multimodal LLMs
The Medical Futurist
JUNE 21, 2025
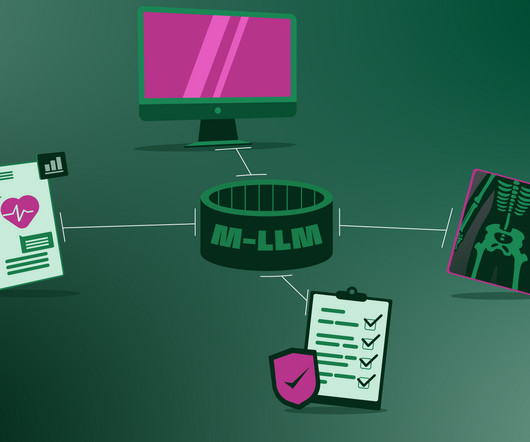
The situation today is as follows: One company manufactures software for the radiology department which use a certain format of AI in their daily work. An average doctor will then be able to easily work with the radiological AI software, the AI software managing the EMRs, and the fourth, and eighth (etc. ) AI used in the hospital.











Let's personalize your content