Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
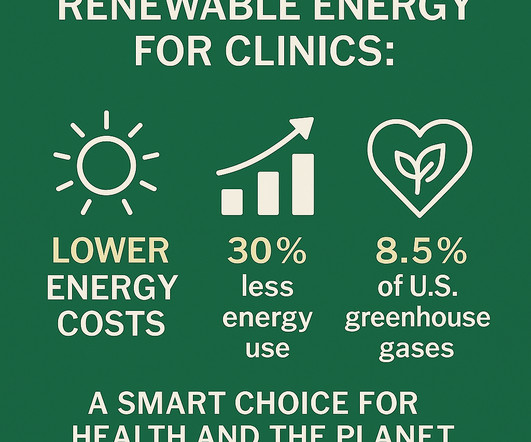
That’s why our Practice Management program includes a guided, meeting-by-meeting path for helping clinics transition to renewable energy and cut utility bills—without overwhelming staff or adding to your to-do list. The breadth of services provided by modern day medicine is profound. Looking for a tailored approach? Cavanagh, D.,













Let's personalize your content