Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
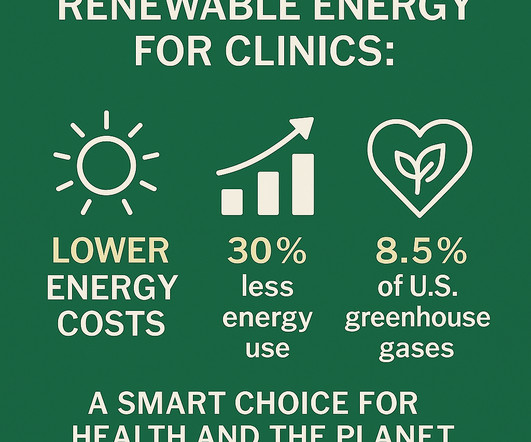
This isn’t just about saving the planet—it’s also about saving money, protecting patients, and strengthening our healthcare systems from the inside out. Latest news & breaking headlines And, in many countries, governments offer financial incentives to install solar panels and even backup batteries. Looking for a tailored approach?










Let's personalize your content