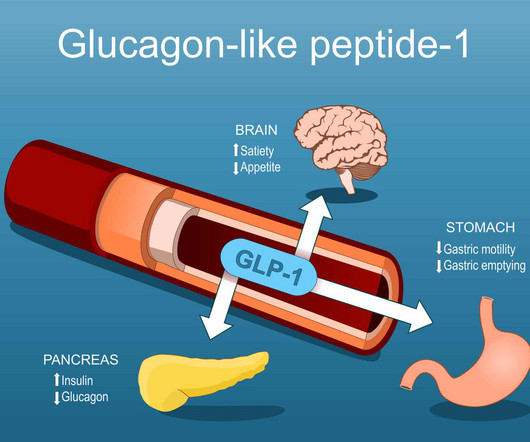
Key Facts GPs Should Know About GLP-1 Analogs
Family Medicine Initiative
APRIL 9, 2024
9 The American Academy of Ophthalmology offers more information here 11. million electronic health records in the USA showed a lower risk for suicidal thoughts in those taking semaglutide than in those taking other weight loss medications. mg is not recommended. Pancreatitis The EMA considers pancreatitis uncommon (0.2%
































Let's personalize your content