AAFP Represents Members During 2025 Legislative Session
Alabama Academy of Family Physicians
MAY 15, 2025
By engaging with lawmakers and key stakeholders, the Academy worked to ensure that the voices of family physicians were heard in discussions that have the potential to impact healthcare delivery and access for communities across the state. What Happened: This legislation has been enacted into law.








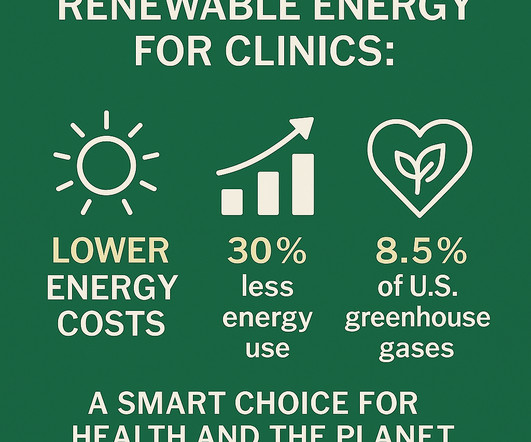

























Let's personalize your content