Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
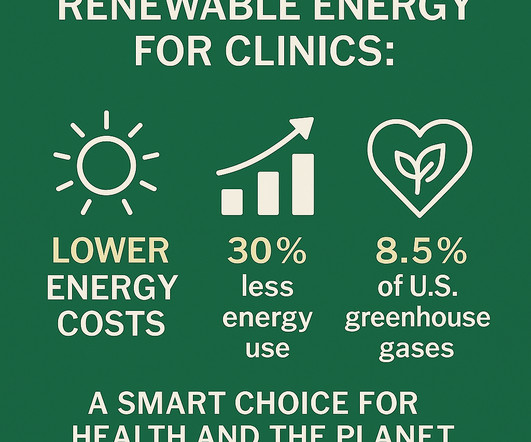
This isn’t just about saving the planet—it’s also about saving money, protecting patients, and strengthening our healthcare systems from the inside out. Dilating and anesthetic eye drops are routinely offered to patients in most ophthalmology clinics. Now, more than ever, they must also become champions of planetary health.
















Let's personalize your content