Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
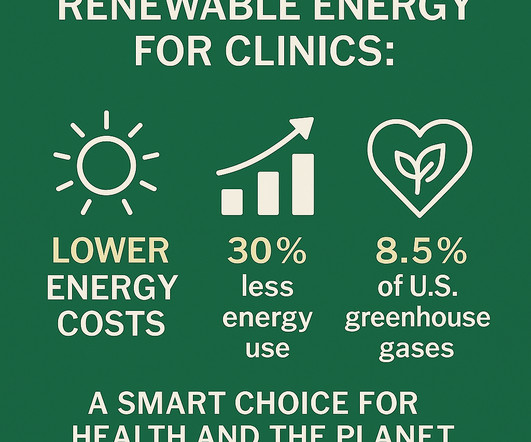
In This Issue : Why Renewable Energy Belongs in Every Clinic Medical Waste: One Model for Improvement OnTrack with your Sustainability Goals? Powering Health, Protecting the Planet – Why Renewable Energy Belongs in Every Clinic Healthcare professionals have always been trusted voices on public health.

















Let's personalize your content