9 Technologies That Will Shape The Future Of Dentistry
The Medical Futurist
JUNE 17, 2025
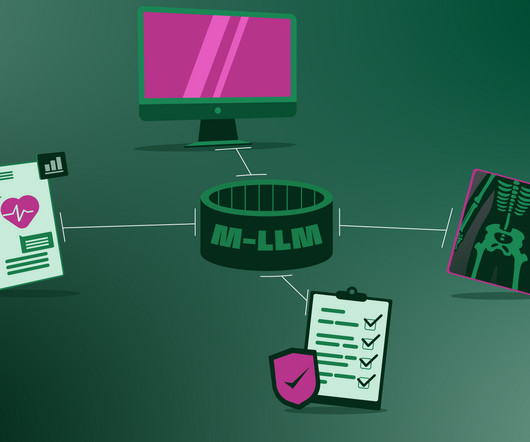
Artificial intelligence Already, dentists employ software to get insights into clinical decision-making, but AI is changing the face of dentistry, just like it is in many other fields. For dentists, it’s transforming diagnosis, decision-making, and treatment planning. And there are so many such devices on the market.




















Let's personalize your content