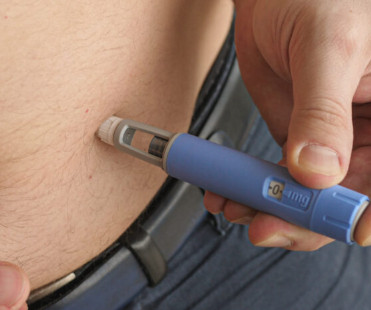
Researchers Propose Solutions to Improve GLP-1 RA Access
Physician's Weekly
JUNE 18, 2025
Other Persistent Barriers & Potential Solutions In another article not presented at the meeting, Stephanie W. “This case highlights the potential for replicable, equity-focused strategies to improve access to obesity care and reduce health disparities,” Dr. Khan and colleagues said. They published their insights in Nature Medicine.










































Let's personalize your content