Researchers Propose Solutions to Improve GLP-1 RA Access
Physician's Weekly
JUNE 18, 2025
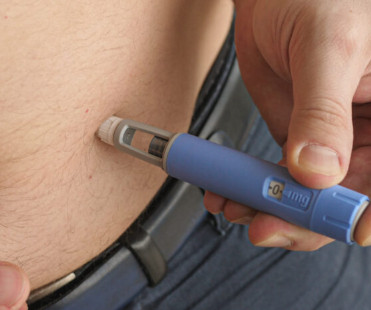
Other Persistent Barriers & Potential Solutions In another article not presented at the meeting, Stephanie W. GLP-1 agonists are effective treatments for weight management, and although regulatory bodies and medical providers are on the right path, we have a long way to go,” the researchers concluded.



















Let's personalize your content