Green Practice News: May 2025
My Green Doctor
MAY 3, 2025
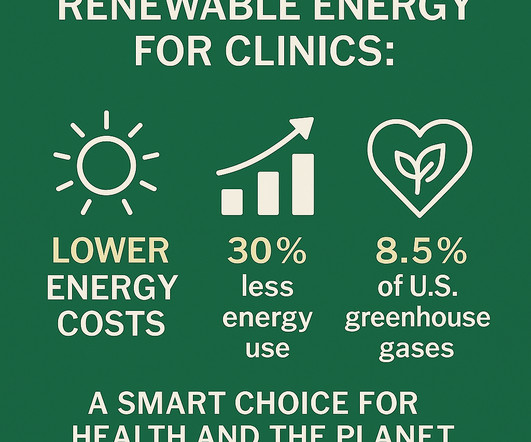
In This Issue : Why Renewable Energy Belongs in Every Clinic Medical Waste: One Model for Improvement OnTrack with your Sustainability Goals? Our Consulting Services offer customized strategies for group practices, health systems, and medical societies ready to accelerate their impact and lead the way in climate-smart care.













Let's personalize your content