Patient experience with Social Prescribing Program in Ontario, Canada [Social determinants and vulnerable populations]
Annals of Family Medicine
NOVEMBER 20, 2024
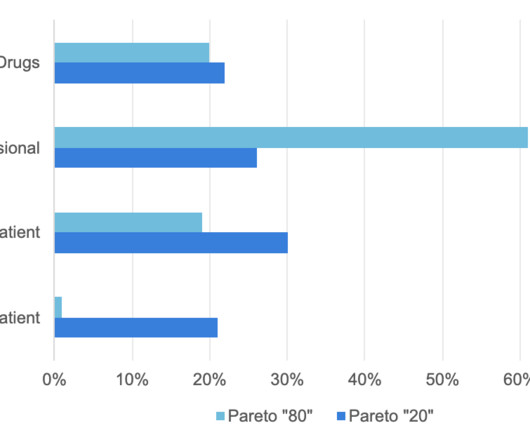
Context Social Prescribing (SP) is an approach to help individuals address their health and social needs wherein a healthcare practitioner refers patients to non-clinal services in the community. Models of SP vary, and the experience of patients across these models is less known. ARC: All (N=17) participants used navigation.
















































Let's personalize your content