Better Care Models Needed for Young Adults With Obesity
Physician's Weekly
JUNE 23, 2025
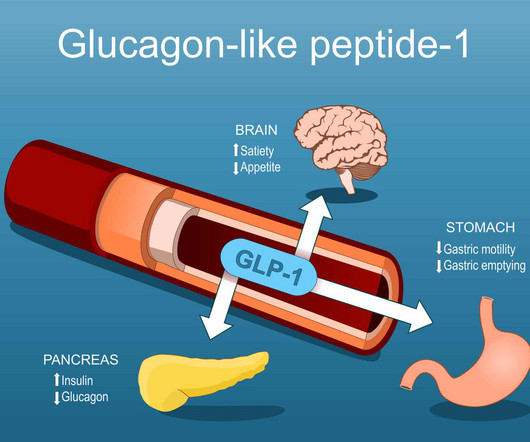
Obesity drugs were not utilized often in young adults and adolescents despite high complication rates, prompting researchers to call for chronic, holistic care. Obesity in children and young adults is associated with the early development of several obesity-related complications,” wrote Theresa Hunter Gibble, PhD , and colleagues.




















Let's personalize your content