Comparing Long-Term Pressure Outcomes in Glaucoma Surgery
Physician's Weekly
JUNE 28, 2025
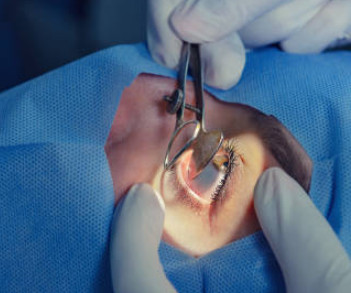
An 11-year cohort study published in June 2025 in the issue of BMC Ophthalmology evaluated the long-term outcomes of trabeculectomy versus canaloplasty (TVC) in individuals with open-angle glaucoma. They followed individuals from the original 2015 TVC cohort. The mean number of glaucoma medications used was 1.0 ± 1.4







































Let's personalize your content