Medicaid expansion is in the eye of the beholder
The Health Policy Exchange
MAY 7, 2013
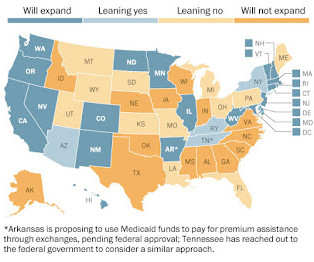
Instead, the Court gave individual states the option to accept or decline the expansion, which, though far more generous with federal matching funds than the existing program, would still require states to spend more within already strapped budgets.
































Let's personalize your content