Medicaid expansion is in the eye of the beholder
The Health Policy Exchange
MAY 7, 2013
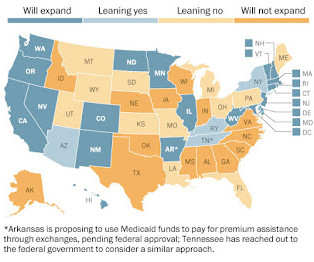
To supporters of the Affordable Care Act, legislative expansion of the Medicaid program is a welcome financial and health care bonanza for states and uninsured patients. In fiscal year 2011, Medicaid spending totaled $414 billion, with two-thirds going to services for disabled elderly persons.



















Let's personalize your content