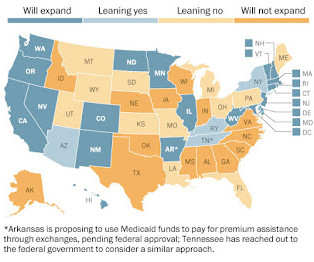
Medicaid expansion is in the eye of the beholder
The Health Policy Exchange
MAY 7, 2013
To the ACA's detractors, Medicaid expansion is a hostile government takeover that must be opposed in principle, regardless of potential benefits of an infusion of federal dollars.

























Let's personalize your content