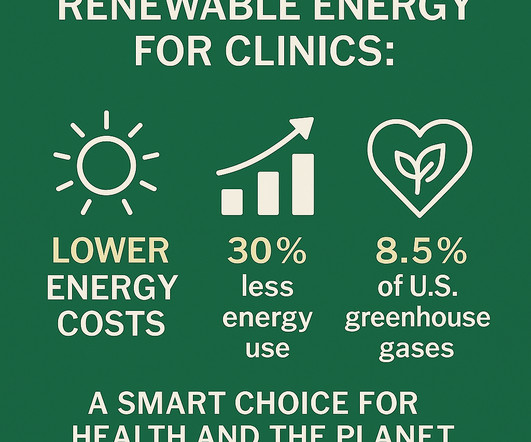
The Rising Tide of Medical Waste: One Model for Improvement
My Green Doctor
MAY 3, 2025
The breadth of services provided by modern day medicine is profound. A 2010 research study found that treating two patients per bottle instead of one would decrease plastic waste by approximately 12.69 The product manufacturers and vendors will need to be active partners as well. Eye 24 , 361–363 (2010). 3 “Each U.S.
















Let's personalize your content